Carbene complexes of phosphorus(v) fluorides substituted with perfluoroalkyl-groups synthesized by oxidative addition. Cleavage of the complexes reveals a new synthetic protocol for ionic liquids†‡
Abstract
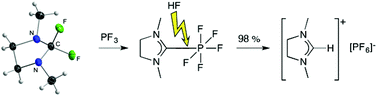
The crystal structures of the previously reported 2,2-difluoro-1,3-dimethylimidazolidine and its acyclic analog were determined by in situ crystallization. Both liquid fluorinating agents have recently been introduced as potent precursors for the synthesis of p-block element carbene complexes. These fluorine compounds were compared with chloro-amidinium chlorides, which are already established carbene precursors. The chlorides were characterized as ion pairs and contrasted to the previous interpretation as charge-transfer complexes between carbene and dichlorine. Furthermore, the two precursors are used for the synthesis of a series of new carbene complexes of phosphorus(V) fluorides substituted with perfluoroalkyl-groups. These unusually stable hexacoordinate phosphorus(V) complexes were treated with anhydrous hydrogen fluoride to yield imidazolium phosphates. This procedure allows the synthesis of salts used as ionic liquids in high yield and high purity.

 Please wait while we load your content...
Please wait while we load your content...