Structural variation in Zn(ii) coordination polymers built with a semi-rigid tetracarboxylate and different pyridine linkers: synthesis and selective CO2 adsorption studies†
Abstract
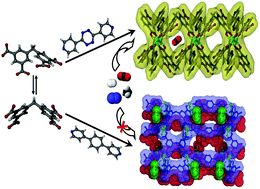
In an effort towards the rational design of porous MOFs with a functionalized channel surface, 3,3′,5,5′-tetracarboxydiphenylmethane (H4L1) has been used in combination with two different bipyridine ligands of similar lengths as linkers, and Zn(II) ions as nodes. Under solvothermal conditions, two Zn(II) coordination polymers, {[Zn(H2L1)(L2)]·DMF·2H2O}n (1) and {[Zn2(L1)(L3)(DMF)2]·DMF·4H2O}n (2) (DMF = dimethyl formamide, L2 = 3,6-di-pyridin-4-yl-[1,2,4,5]tetrazine, L3 = 4,4′-bispyridylphenyl) are formed in moderate yields. The obvious kink in the central methylene spacer of H4L1 induces either C2v or Cs symmetry in the ligand, allowing different architectures in the resulting frameworks. Single crystal X-ray analysis shows that compound 1 is a one-dimensional (1D) double chain architecture with rhombus voids, linked by Zn2(CO2)4 paddle-wheel secondary building units (SBUs). The tetrazine and pyridine moieties of the co-ligand and free carboxylic acid groups are lined along the voids of the framework. Compound 2, on the other hand, crystallizes as an infinite two-dimensional corrugated sheet structure, where individual sheets are stacked in —ABAB— patterns along the crystallographic b-axis. Thermogravimetric analysis (TGA) and variable temperature powder X-ray diffraction (VTPXRD) studies reveal high thermal stability for 1 but 2 collapses soon after desolvation. The desolvated framework 1′ shows selective CO2 adsorption over N2, H2, and CH4 at 273 K, with an isosteric heat of CO2 adsorption of 21.3 kJ mol−1, suggesting an interaction of the CO2 molecules with the channel walls.

 Please wait while we load your content...
Please wait while we load your content...