New di-ferrocenyl-ethynylpyridinyl triphenylphosphine copper halide complexes and related di-ferricenyl electro-crystallized materials†
Abstract
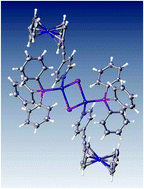
Three new neutral di-ferrocenyl-ethynylpyridinyl copper complexes, [L2(CuCl)2(PPh3)2] (2), [L2(CuBr)2(PPh3)2] (3), and [L2(CuI)2(PPh3)2] (4) were synthesized from the ferrocenyl-ethynylpyridine ligand (L) (1), the appropriate copper halide CuX (with X = Cl−, Br−, I−) and triphenylphosphine. These neutral complexes were fully characterized by spectroscopic methods and by single crystal X-ray crystallography. Cyclic voltammetry in dichloroethane revealed chemically reversible ferrocenyl oxidation signals followed by characteristic “stripping reduction peaks” showing evidence for oxidation-product electro-crystallization. Scanning electron microscopy confirmed spontaneous formation of crystalline oxidation products with three distinct morphologies for X = Cl−, Br−, I−. Energy dispersive X-ray elemental analysis data show Fe : P ratios of 1 : 2.0, 1 : 2.1 and 1 : 2.1 for electro-crystallization products of complexes 2, 3, and 4, respectively, indicating the presence of two [PF6]− anions in the vicinity of the dioxidized complexes, and suggesting product formulae [2]2+[PF6]−2, [3]2+[PF6]−2 and [4]2+[PF6]−2.

 Please wait while we load your content...
Please wait while we load your content...