Phenanthroline ligands are biologically more active than their corresponding ruthenium(ii) arene complexes†
Abstract
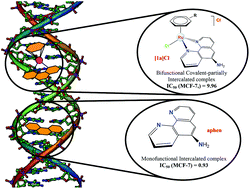
New cationic, half-sandwich Ru(II) arene compounds of general formula [(η6-arene)RuCl(κ2-N,N-L)]X (where L are functionalized phenanthrolines such as 1,10-phenanthroline-5-amine (aphen); 5,6-epoxy-5,6-dihydro-[1,10]phenanthroline (ephen); or 4,7-dihydroxy-1,10-phenanthroline (dhphen)) have been prepared to study their anticancer potential. All the isolated complexes have been fully characterized by spectroscopic and analytical techniques. The structure of endo-[(η6-p-cymene)RuCl(κ2-N,N-ephen)]BF4, [2a](BF4), has been determined by X-ray crystallography. The in vitro cytotoxicity of the aphen and ephen phenanthrolines and their Ru derivatives [(η6-p-cymene)RuCl(κ2-N,N-L)]Cl ([1a]Cl and [2a]Cl, respectively) assessed in tumour cell lines has shown that the free ligands are more active than the organometallic products, with aphen being the most potent specimen. Furthermore, the binding interaction of both [1a]Cl and aphen with calf thymus DNA (CT-DNA) has been investigated using a variety of thermodynamic and kinetic techniques. The aphen free ligand intercalates into DNA at low ligand content, whereas [1a]Cl forms with DNA a bifunctional partially intercalated-covalent complex, in which the intercalation constant is nearly three orders of magnitude lower than that of aphen. This finding demonstrates that the covalent binding noticeably weakens the intercalation, a feature presumably related to the higher cytotoxic activity of aphen relative to that of [1a]Cl.

 Please wait while we load your content...
Please wait while we load your content...