Luminescent alkynyl-gold(i) coumarin derivatives and their biological activity†
Abstract
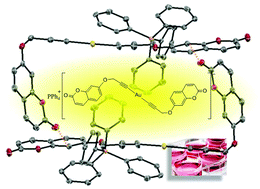
The synthesis and characterization of three propynyloxycoumarins are reported in this work together with the formation of three different series of gold(I) organometallic complexes. Neutral complexes are constituted by water soluble phosphines (PTA and DAPTA) which confer water solubility to them. The X-ray crystal structure of 7-(prop-2-in-1-yloxy)-1-benzopyran-2-one and its corresponding dialkynyl complex is also shown and the formation of rectangular dimers for the gold derivative in the solid state can be observed. A detailed analysis of the absorption and emission spectra of both ligands and complexes allows us to attribute the luminescent behaviour to the coumarin organic ligand. Moreover, the presence of the gold(I) metal atom seems to be responsible for an increase of coumarin phosphorescence emission. The biological activity of the complexes showed that the anionic complexes triggered strong cytotoxic effects in two different cell lines whereas the neutral gold alkynyl complexes led to lower effects against tumor cell growth. Thioredoxin reductase (TrxR) inhibition was very strong in the case of the neutral complexes (IC50 values below 0.1 μM) but moderate for the anionic complexes (IC50 values above 0.8 μM).
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...