Ring expansion of a 2-rhodaoxetane: insertion chemistry with unsaturated molecules†
Abstract
A 2-rhodaoxetane was found to react with unsaturated electrophiles, such as highly electron-deficient acetylene dicarboxylates, CS2 and various aldehydes, to form a series of six-membered metallacycles. These metallacycles were characterized by 1H, 13C and 2D NMR spectroscopic techniques, as well as HRMS and, for one complex, XRD. In some cases, the insertions were found to be reversible.
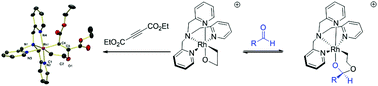

 Please wait while we load your content...
Please wait while we load your content...