Solvent-controlled synthesis of tetranuclear cage-like copper(ii) silsesquioxanes. Remarkable features of the cage structures and their high catalytic activity in oxidation with peroxides†
Abstract
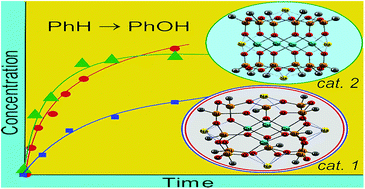
Two principally different in their molecular architecture isomeric tetranuclear copper(II) silsesquioxanes, “Globule”-like compound [(PhSiO1.5)12(CuO)4(NaO0.5)4] (1) and “Sandwich”-like derivative [(PhSiO1.5)6(CuO)4(NaO0.5)4(PhSiO1.5)6] (2), were synthesized by the partial cleavage of polymeric copper(II) silsesquioxane [(PhSiO1.5)2(CuO)]n by tetraphenylcyclotetrasiloxanolate. The route leading to the formation of either 1 or 2 entirely depends on the nature and composition of the solvent used for this reaction. Thus, the process in an ethanol–1-butanol solution gives compound 1. When a 1,4-dioxane–methanol mixture was used, compound 2 was prepared. The structures and unusual crystal packing of the cages were confirmed by the X-ray studies. It has been found that the reaction of benzene with H2O2 in acetonitrile solution at 50 °C catalyzed by 1 requires addition of trifluoroacetic acid (TFA) in low concentration and gives phenol with a turnover number (TON) of 250 after 3 h. The initial reaction rate W0 linearly depends on the concentration of catalyst 2. The oxidation of 1-phenylethanol to acetophenone with hydrogen peroxide catalyzed by complex 1 in the presence of TFA is not efficient. In contrast, 1 exhibited excellent activity in the oxidation with tert-butyl hydroperoxide (TBHP) in the absence of any acid (the yield of acetophenone was close to the quantitative, TON attained 475 after 2 h). A kinetic study of this reaction led to the conclusion that the process occurs with the participation of radicals tert-BuO˙ produced in the Cu-promoted decomposition of TBHP. The mode of dependence of W0 on the initial concentration of TBHP indicates the formation of an intermediate adduct between the catalyst 1 and TBHP (characterized by the equilibrium constant K1 ≈ 2 M−1 for the conditions of conducted experiments) followed by subsequent decomposition of the adduct (k2 ≈ 0.2 s−1) to generate an intermediate species tert-BuO˙ which induces the alcohol oxidation.

 Please wait while we load your content...
Please wait while we load your content...