Programming MIL-101Cr for selective and enhanced CO2 adsorption at low pressure by postsynthetic amine functionalization†
Abstract
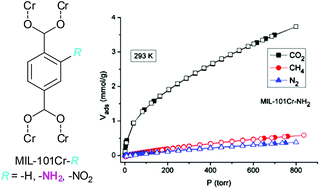
MIL-101Cr fully or partially (p) postsynthetically modified with nitro (–NO2) or amino (–NH2) groups was shown to be a robust, water stable, selective and enhanced carbon dioxide (CO2) adsorption material with the amine-functionality. The highly microporous amine-modified frameworks (up to 1.6 cm3 g−1 total pore volume) exhibit excellent thermal stability (>300 °C) with BET surface areas up to 2680 m2 g−1. At 1 bar (at 273 K) the gases CO2, CH4 and N2 are adsorbed up to 22.2 wt%, 1.67 wt% and 2.27 wt%, respectively. The two amine-modified MIL-101Cr-NH2 (4) and MIL-101Cr-pNH2 (5) showed the highest gas uptake capacities in the series with high ratios for the CO2 : N2 and CO2 : CH4 selectivities (up to 119 : 1 and 75 : 1, respectively, at 273 K). Comparison with non-modified MIL-101Cr traces the favorable CO2 adsorption properties of MIL-101Cr-NH2 (4) and MIL-101Cr-pNH2 (5) to the presence of the Lewis-basic amine groups. MIL-101Cr-NH2 (4) has a high isosteric heat of adsorption of 43 kJ mol−1 at zero surface coverage and also >23 kJ mol−1 over the entire adsorption range, which is well above the heat of liquefaction of bulk CO2. Large CO2 uptake capacities of amine-functionalized 4 and 5, coupled with high adsorption enthalpy, high selectivities and proven long-term water stability, make them suitable candidates for capturing CO2 at low pressure from gas mixtures including the use as a CO2 sorbent from moist air.

 Please wait while we load your content...
Please wait while we load your content...