Biomimetic iron(iii) complexes of facially and meridionally coordinating tridentate 3N ligands: tuning of regioselective extradiol dioxygenase activity in organized assemblies†
Abstract
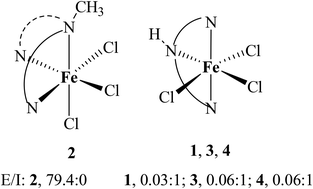
Four mononuclear iron(III) complexes of the type [Fe(L)Cl3] 1–4, where L is a tridentate 3N ligand such as (2-pyridin-2-ylethyl)(pyridin-2-ylmethyl)amine (L1), (methyl)(2-pyridin-2-ylethyl)(pyridin-2-ylmethyl)amine (L2), bis(pyridin-2-ylethyl)amine (L3), and (1-methyl-1H-imidazol-2-ylmethyl)(pyridin-2-ylethyl)amine (L4), have been isolated and studied as functional models for catechol dioxygenase enzymes. In [Fe(L2)Cl3] 2, the ligand L2 is coordinated facially to iron(III) whereas in [Fe(L1)Cl3] 1 and [Fe(L4)Cl3] 4 the ligands L1 and L4 are coordinated meridionally. In DCM, CH3CN and aqueous SDS, CTAB and TX-100 micellar media, the positions of both the low and high energy catecholate-to-iron(III) LMCT bands (465–530, 690–860 nm) observed for the 3,4-di-tert-butylcatecholate (DBC2−) adducts of the iron(III) complexes vary in the order 2 > 1 > 3 > 4, which reflects the influence of the stereoelectronic factors, mode of coordination and the chelate ring size formed by the tridentate ligands. Spectral and electrochemical studies disclose the formation and location of the cationic adducts as solvated [Fe(L)(DBC)(H2O)]+ species mostly in the aqueous micellar pseudophases of SDS and TX-100 and in the aqueous phase of CTAB micellar solution. The [Fe(L)(DBC)Cl] adducts of 1, 3 and 4, generated in situ, afford major amounts of intradiol cleavage products (17.0–70.0%) and smaller amounts of extradiol (1.2–4.2%) products with varying extradiol to intradiol cleavage product selectivity (E/I: 1, 0.08 : 1; 3, 0.02 : 1; 4, 0.3 : 1). On the other hand, interestingly, the adduct [Fe(L2)(DBC)Cl] of 2 generated in DCM yields a major amount of extradiol (54.0%) and a lower amount (18.3%) of the intradiol cleavage products (E/I, 3 : 1). Remarkably, in aqueous SDS micellar media, it shows exclusive extradiol cleavage products (79.4%) while all the other complexes show very low selectivity (E/I: 1, 0.03 : 1; 2, 79.4 : 0, 3, 0.06 : 1, 4, 0.06 : 1), suggesting the suitability of SDS medium for 2 to elicit exclusive extradiol cleavage. The TX-100 micellar medium also provides a suitable hydrophobic environment for 2 to elicit extradiol cleavage. However, in CTAB micellar medium, 2 shows cleavage selectivity lower than others. Also, the rate of dioxygenation is higher in SDS micellar medium than in DCM, and is dependent upon the chelate ring size.

 Please wait while we load your content...
Please wait while we load your content...