2-(1-(2-Benzhydrylnaphthylimino)ethyl)pyridylnickel halides: synthesis, characterization, and ethylene polymerization behavior†
Abstract
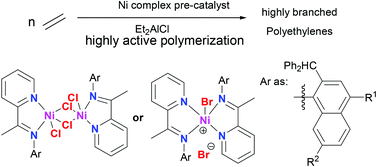
A series of 2-(1-(2-benzhydrylnaphthylimino)ethyl)pyridine derivatives (L1–L3) was synthesized and fully characterized. The organic compounds acted as bi-dentate ligands on reacting with nickel halides to afford two kinds of nickel complexes, either mononuclear bis-ligated L2NiBr2 (Ni1–Ni3) or chloro-bridged dinuclear L2Ni2Cl4 (Ni4–Ni6) complexes. The nickel complexes were fully characterized, and the single crystal X-ray diffraction revealed for Ni2, a distorted square pyramidal geometry at nickel comprising four nitrogens of two ligands and one bromide; whereas for Ni4, a centrosymmetric dimer possessing a distorted octahedral geometry at nickel was formed by two nitrogens of one ligand, two bridging chlorides and one terminal chloride along with oxygen from methanol (solvent). When activated with diethylaluminium chloride (Et2AlCl), all nickel complexes performed with high activities (up to 1.22 × 107 g (PE) mol−1 (Ni) h−1) towards ethylene polymerization; the obtained polyethylene possessed high branching, low molecular weight and narrow polydispersity, suggestive of a single-site active species. The effect of the polymerization parameters, including the nature of the ligands/halides on the catalytic performance is discussed.

 Please wait while we load your content...
Please wait while we load your content...