Carbohydrate-appended photocytotoxic (imidazophenanthroline)-oxovanadium(iv) complexes for cellular targeting and imaging†
Abstract
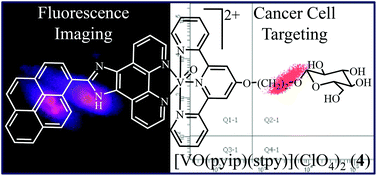
Oxovanadium(IV) complexes [VO(aip)(L)](ClO4)2 (L = phtpy, 1; stpy, 2) and [VO(pyip)(L)](ClO4)2 (L = phtpy, 3; stpy, 4), where aip is 2-(9-anthryl)-1H-imidazo[4,5-f][1,10]phenanthroline, pyip is [2-(1-pyrenyl)-1H-imidazo[4,5-f][1,10]phenanthroline, phtpy is (4′-phenyl)-2,2′:6′,2′′-terpyridine and stpy is (2,2′:6′,2′′-terpyridin-4′-oxy)ethyl-β-D-glucopyranoside, were prepared, characterized and their DNA binding and photocleavage activity, cellular uptake and photocytotoxicity in visible light were studied. The complexes are avid binders to calf thymus DNA (Kb ∼105 mol−1). They efficiently cleave pUC19 DNA in red light of 705 nm via the formation of HO˙ species. The glucose appended complexes 2 and 4 showed higher photocytotoxicity in HeLa and Hep G2 cells over the normal HEK 293T cells. No such preference was observed for the phtpy complexes 1 and 3. No significant difference in IC50 values was observed for the HEK 293T cells. Cell cycle analysis showed that the glucose appended complexes 2 and 4 are more photocytotoxic in cancer cells than in normal cells. Fluorescence microscopy was done to study the cellular localization of complex 4 having a pendant pyrenyl group.

 Please wait while we load your content...
Please wait while we load your content...