Trinuclear heterometallic CuII–MnII complexes of a salen type Schiff base ligand: anion dependent variation of phenoxido bridging angles and magnetic coupling†
Abstract
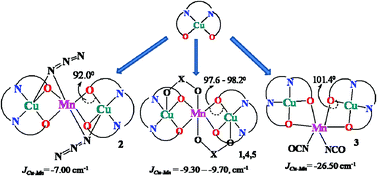
Five new trinuclear heterometallic CuII–MnII complexes [(CuL)2Mn(O2CPh)2] (1), [(CuL)2Mn(N3)2] (2), [(CuL)2Mn(NCO)2] (3), [(CuL)2Mn(NO3)2] (4) and [(CuL)2Mn(Sal)2]·CH2Cl2 (5) have been synthesized with the di-Schiff base ligand H2L (where H2L = N,N′-bis(salicylidene)-1,3-propanediamine and Sal = salicylate). These complexes with different anionic co-ligands have been synthesized to attain a large variation in phenoxido bridging angles and to investigate its consequence on magnetic properties. Single crystal X-ray diffraction analyses reveal that complexes 1, 2, 4 and 5 are linear, whereas 3 has an angular geometry. Variable temperature magnetic susceptibility measurements suggest that all five complexes possess an overall antiferromagnetic interaction between CuII and MnII ions, which results in a final ferrimagnetic ground state with spin 3/2 in the CuII–MnII–CuII trinuclear structure. The weakest antiferromagnetic interaction (JCu–Mn = −7.0 cm−1) is observed for 2 having the lowest value of the Cu–O–Mn angle (92.0°), while the strongest antiferromagnetic interaction (JCu–Mn = −26.5 cm−1) is observed for 3 having the largest Cu–O–Mn angle (101.4°). Complexes 1, 4 and 5 show average Cu–O–Mn angles of 98.2°, 97.6° and 97.7°, respectively, that lead to intermediate antiferromagnetic interactions (JCu–Mn = −9.6, −9.7, −9.3 cm−1 respectively).

 Please wait while we load your content...
Please wait while we load your content...