The coordination chemistry of tartronic acid with copper: magnetic studies of a quasi-equilateral tricopper triangle†
Abstract
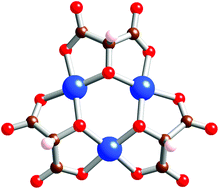
The coordination chemistry of tartronic acid, 1, with copper(II) has been investigated. Structures of two complexes are reported containing respectively the complex [Cu(1–2H)2Cl]3− where 1 acts as a bidentate ligand through carboxylates, and [Cu3(1–3H)3]3− where the alcohol function is deprotonated to bridge two coppers in a triangular trinuclear complex. The latter species undergoes facile oxidation leading to carbon–carbon bond formation. The magnetic and EPR properties of the trinuclear complex have been studied in detail.

 Please wait while we load your content...
Please wait while we load your content...