Tuning the olefin epoxidation by manganese(iii) complexes of bisphenolate ligands: effect of Lewis basicity of ligands on reactivity†
Abstract
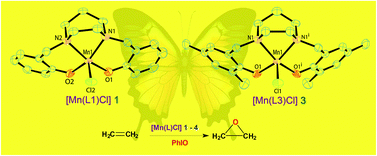
A new family of manganese(III) complexes of the type [Mn(L)Cl], where H2L is 1,4-bis(2-hydroxy-benzyl)-1,4-diazepane (H2(L1)), 1,4-bis(2-hydroxy-4-methylbenzyl)-1,4-diazepane (H2(L2)), 1,4-bis(2-hydroxy-3,5-dimethylbenzyl)-1,4-diazepane (H2(L3)) and 1,4-bis(2-hydroxy-3,5-di-tert-butylbenzyl)-1,4-diazepane (H2(L4)), has been isolated and studied as a catalyst for epoxidation reaction. Complexes 1–4 have been characterized using elemental analysis, electronic spectral and electrochemical methods and ESI-MS. The single crystal X-ray structures of 1 and 3 contain the MnN2O2Cl chromophore with a novel square pyramidal coordination geometry (τ: 1, 0.11; 3, 0.00). All the complexes possess a distorted square pyramidal coordination geometry in solution, as revealed by the characteristic bands observed in the electronic spectra. A time dependent density functional theory (TD-DFT) calculation has been performed to assist in the assignment of the electronic absorption spectral bands of the complexes. The Mn(III)/Mn(II) redox potentials (E1/2) of 1–4 fall within the narrow range of 0.279–0.320 V. The catalytic ability of the complexes towards olefin epoxidation has been investigated using PhIO as the oxygen source at room temperature under an N2 atmosphere. Addition of N-methylimidazole to the reaction mixture leads to an increase in the epoxide yield. A correlation between the Lewis acidity of the Mn(III) center as tuned by the substituents on the phenolate ligand, and the epoxide yield and product selectivity has been observed. The present complexes act as better chemoselective catalysts for epoxidation of cyclohexene and styrene rather than cyclooctene.

 Please wait while we load your content...
Please wait while we load your content...