A radical pathway in catecholase activity with nickel(ii) complexes of phenol based “end-off” compartmental ligands†
Abstract
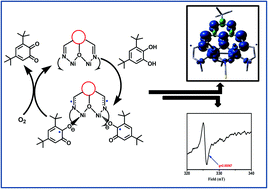
Seven dinuclear and one dinuclear based dicyanamide bridged polymeric NiII complexes of phenol based compartmental ligands (HL1–HL4) have been synthesized with the aim to investigate their catecholase-like activity and to evaluate the most probable mechanistic pathway involved in this process. The complexes have been characterized by routine physicochemical studies as well as by X-ray single crystal structure analyses namely [Ni2(L2)(SCN)3(H2O)(CH3OH)] (1), [Ni2(L4)(SCN)3(CH3OH)2] (2), [Ni2(L2)(SCN)2(AcO)(H2O)] (3), [Ni2(L4)(SCN)(AcO)2] (4), [Ni2(L2)(N3)3(H2O)2] (5), [Ni2(L4)(N3)3(H2O)2] (6), [Ni2(L1)(AcO)2(N(CN)2)]n (7) and [Ni2(L3)(AcO)2(N(CN)2)] (8), [SCN = isothiocyanate, AcO = acetate, N3 = azide, and N(CN)2 = dicyanamide anion; L1–4 = 2,6-bis(R2-iminomethyl)-4-R1-phenolato, where R1 = methyl and tert-butyl, R2 = N,N-dimethyl ethylene for L1–2 and R1 = methyl and tert-butyl, R2 = 2-(N-ethyl) pyridine for L3–4]. A UV-vis spectrophotometric study using 3,5-di-tert butylcatechol (3,5-DTBC) reveals that all the complexes are highly active in catalyzing the aerobic oxidation of (3,5-DTBC) to 3,5-di-tert-butylbenzoquinone (3,5-DTBQ) in methanol medium with the formation of hydrogen peroxide. An EPR study confirms the generation of radicals during the catalysis. Cyclic voltammetric studies of the complexes in the presence and absence of 3,5-DTBC have been performed. Reduction of NiII to NiI and that of the imine bond of the ligand system have been detected at ∼−1.0 V and ∼−1.5 V, respectively. Coulometric separation of the species at −1.5 V followed by the EPR study at 77 K confirms the species as an organic radical and thus most probably reduced imine species. Spectroelectrochemical analysis at −1.5 V clearly indicates the oxidation of 3,5-DTBC and thus suggests that the radical pathway is supposed to be responsible for the catecholase-like activity exhibited by the nickel complexes. The ligand centred radical generation has further been verified by density functional theory calculation.

 Please wait while we load your content...
Please wait while we load your content...