Heterobimetallic dual-catalyst systems for the hydrolytic kinetic resolution of terminal epoxides†
Abstract
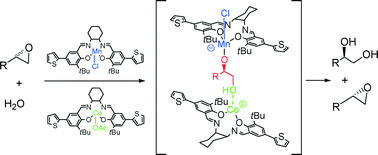
A heterobimetallic dual-catalyst system based on the preparation and use of various salen complexes has been developed for the hydrolytic kinetic resolution (HKR) of terminal epoxides. A combination of cobalt-salen and manganese-salen complexes, generated from ligands with the same configuration possessing thiophene or pyrrole moieties, produced indeed highly selective catalysts for the hydrolysis of epibromohydrin. This effect could also be extended to other terminal epoxides and to the more challenging ring opening of cyclohexene oxide. Kinetic studies indicated that only one CoIII salen complex was involved in the rate-determining step, which supported a heterobimetallic highly enantioselective pathway based on the crucial existence of in situ generated CoIII-OH species, previously postulated in the literature. The beneficial effect of the presence of additional Mn-complexes was ascribed to the inhibition of the alternative less enantioselective monometallic reaction pathway by epoxide activation.

 Please wait while we load your content...
Please wait while we load your content...