Catalytic oxidation of formic acid by dioxygen with an organoiridium complex†
Abstract
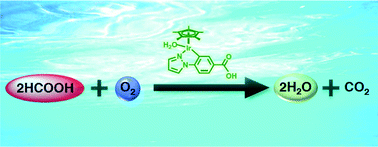
Catalytic oxidation of formic acid by dioxygen occurred efficiently using an organoiridium complex ([IrIII(Cp*)(4-(1H-pyrazol-1-yl-κN2)benzoic acid-κC3)(H2O)]2SO4, 1) as a catalyst in a water-containing organic solvent as well as in water at ambient temperature. The catalytic cycle is composed of the reduction of 1 by formate to produce the hydride complex, which reduces dioxygen to water to regenerate 1.

 Please wait while we load your content...
Please wait while we load your content...