Reactivity of bromofluorenes in palladium-catalysed direct arylation of heteroaromatics
Abstract
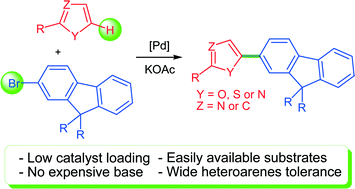
The palladium-catalysed direct arylation using bromofluorenes and heteroaromatics as the coupling partners proceeded in moderate to high yields using only 0.1–0.5 mol% Pd(OAc)2 or 1 mol% PdCl(C3H5)(dppb) as the catalyst and KOAc as the base. A wide variety of heteroarenes have been successfully employed, allowing their properties to be easily tuned. From 2,7-dibromofluorene, successive arylations allow the introduction of two different heteroarenes at carbons C2 and C7.

 Please wait while we load your content...
Please wait while we load your content...