Transition metal-rich mesoporous silicas and their enhanced catalytic properties†
Abstract
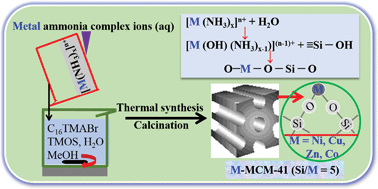
A controllable and simple direct hydrothermal synthesis route was designed for synthesizing well-ordered mesoporous silica incorporating transition metals (M) (Ni, Cu, Zn, Co) with high metal loading. M-incorporated mesoporous silica could be obtained from a starting synthesis mixture with a Si/M mole ratio of 5 using transition metal–ammonia (NH3) complex ions [M(NH3)x]n+ as base. The Si/M mole ratio of 5 is the lowest value yet reported. XPS, UV-vis and H2-TPR analyses demonstrated that a chemical bond was formed between metal and silicon via oxygen and no bulk metal oxides existed in any of the M-MCM-41 samples; in other words, only tetrahedral coordinated metal species were detected. The formation of –O–M–O–Si–O– is completed via the reaction between hydrolyzate [M(OH)(NH3)x−1](n−1)+ from [M(NH3)x]n+ and ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–OH (silanol sites) from a silica source (tetramethoxysilane (TMOS)). All the M-MCM-41 samples possessed remarkable physical properties and thermal stability. Ni-MCM-41, Cu-MCM-41 and CoMCM-41 catalysts exhibited excellent catalytic efficiency for carbon dioxide (CO2) hydrogenation, although Zn-MCM-41 catalyst did not. Ni-MCM-41 catalyst suited methanation, resulting in high CO2 conversion rate and methane selectivity, while Cu-MCM-41 catalyst favored the reverse water gas shift (RWGS) reaction and realized high CO2 conversion rate to carbon monoxide. A kinetic study was also carried out for methanation and RWGS reaction. Using Ni-MCM-41 catalyst for methanation, the rate equation could be expressed as r = kCCO20.68CH23.31, where C represents concentration. Using Cu-MCM-41 catalyst for RWGS reaction, the rate equation could be expressed as r = kCCO20.5CH21.1, where C represents concentration.
Si–OH (silanol sites) from a silica source (tetramethoxysilane (TMOS)). All the M-MCM-41 samples possessed remarkable physical properties and thermal stability. Ni-MCM-41, Cu-MCM-41 and CoMCM-41 catalysts exhibited excellent catalytic efficiency for carbon dioxide (CO2) hydrogenation, although Zn-MCM-41 catalyst did not. Ni-MCM-41 catalyst suited methanation, resulting in high CO2 conversion rate and methane selectivity, while Cu-MCM-41 catalyst favored the reverse water gas shift (RWGS) reaction and realized high CO2 conversion rate to carbon monoxide. A kinetic study was also carried out for methanation and RWGS reaction. Using Ni-MCM-41 catalyst for methanation, the rate equation could be expressed as r = kCCO20.68CH23.31, where C represents concentration. Using Cu-MCM-41 catalyst for RWGS reaction, the rate equation could be expressed as r = kCCO20.5CH21.1, where C represents concentration.

 Please wait while we load your content...
Please wait while we load your content...