Nitrile reductase as a biocatalyst: opportunities and challenges
Abstract
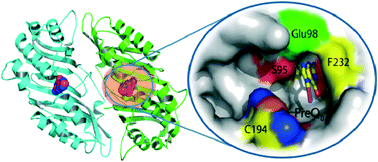
Nitrile-containing compounds are widely manufactured and extensively used in the chemical and pharmaceutical industries as synthetic intermediates or precursors. Nitrile hydratase and nitrilase have been successfully developed as biocatalysts for the production of amides and carboxylic acids from nitrile precursors. The discovery of a family of nitrile reductases that catalyse the reduction of nitrile to amine raised the hope of developing environmentally sustainable nitrile-reducing biocatalysts to replace metal hydride catalysts. However, ten years after the discovery of the QueF nitrile reductases, little progress has been made towards the development of nitrile reductase biocatalysts with altered or broadened substrate specificity. In this article, we analyse and review the structure and catalytic mechanism of QueF nitrile reductases and other structurally related T-fold family enzymes. We argue that the poor evolvability of the T-fold enzymes and the kinetically sluggish reaction catalysed by QueFs pose formidable challenges for developing this family of enzymes into practically useful biocatalysts. The challenges do not seem to be mitigated by current computational design or directed-evolution methods. Searching for another family of nitrile reductases or engineering a more evolvable protein scaffold to support the nitrile-reducing chemistry may be a more viable strategy to develop a nitrile reductase biocatalyst despite another set of foreseeable challenges.

 Please wait while we load your content...
Please wait while we load your content...