The performance of Ag doped V2O5–TiO2 catalyst on the catalytic oxidation of gaseous elemental mercury
Abstract
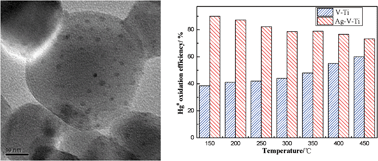
To improve the catalytic oxidation ability for gaseous elemental mercury (Hg0), silver was introduced to V2O5–TiO2 catalysts. The catalysts were prepared by an impregnation method with various additives to obtain well distributed silver nanoparticles on the carrier. It was found that doping silver onto V2O5–TiO2 can significantly improve the catalytic oxidation efficiency of Hg0, and the redox temperature range for Hg0 oxidation was enlarged markedly (150–450 °C). The addition of polyvinylpyrrolidone (PVP) during the preparation of the catalysts can improve the dispersion of silver nanoparticles more effectively, which resulted in a higher Hg0 oxidation efficiency up to 90%. However, the oxidation of Hg0 on the catalyst was slightly inhibited due to the larger silver nanoparticles when the ionic liquid (IL) [bmim][BF4] was used as the additive. The characterization results indicated that V can be induced to a higher oxidation state in the presence of silver nanoparticles, and the transformation trend of TiO2 from the anatase to rutile phase caused by Ag can be minimized in the presence of PVP or ILs. Meanwhile, the mechanisms of the elemental mercury oxidation at various temperature ranges were discussed.

 Please wait while we load your content...
Please wait while we load your content...