Scaling relations between adsorption energies for computational screening and design of catalysts
Abstract
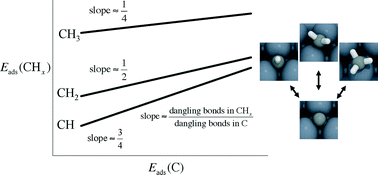
Adsorption energies have significant value as predictors of catalytic performance. An important method of increasing efficiency of adsorption energy calculations is to employ scaling relations, which are linear relationships between adsorption energies of similar adsorbates. They are most commonly used to unify the description of adsorbates that bind to the surface through a particular type of atom. In this work, we review the development and applications of scaling relations. Scaling relations have been observed for a variety of adsorbates bonding through C, O, H, N, and S atoms to the surfaces of transition metals, oxides, nitrides, sulfides, carbides, and nanoparticles. They can be used to increase the efficiency of predictions, simplify descriptions of surface reactivity, and sometimes to derive limits on the effectiveness of a catalyst. Because scaling relations can impose a significant limitation on catalyst design, it is also useful to explore how to design active sites that significantly deviate from them. We discuss applications to a variety of processes, including methane reforming and synthesis, alcohol decomposition and synthesis, electrochemical systems, and conversion of biomass derivatives.
- This article is part of the themed collection: Catalysis in the USA

 Please wait while we load your content...
Please wait while we load your content...