Selective formation of α,ω-ester amides from the aminocarbonylation of castor oil derived methyl 10-undecenoate and other unsaturated substrates†
Abstract
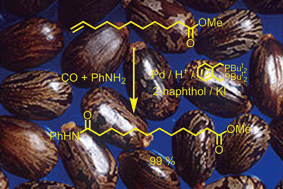
The reaction of long chain alkenes with CO and aniline in the presence of palladium complexes of 1,2-bis-(ditertbutylphosphinomethyl)benzene produces amides with high linear selectivity, with much higher rates and catalyst stability when 2-naphthol and sodium or potassium iodide are added. Unsaturated esters including methyl 10-undecenoate from castor oil give α,ω-ester amides, whilst reactions in THF give N-phenylpyrrolidine.
- This article is part of the themed collection: Sustainable catalytic conversions of renewable substrates

 Please wait while we load your content...
Please wait while we load your content...