Organocatalytic strategies for enantioselective metal-free reductions
Abstract
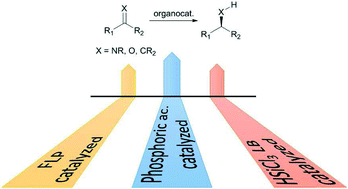
One of the most important chemical transformations is the reduction of multiple bonds, carbon–carbon as well as carbon–heteroatom double bonds, since it leads very often to the generation of new stereocenters in the molecule. The replacement of metal-based catalysts with equally efficient metal-free counterparts is very appealing in view of possible future applications of non toxic, low cost, and environmentally friendly promoters on an industrial scale. This perspective will focus specially, but not exclusively, on the enantioselective reduction of the carbon nitrogen double bond; despite the historical need for and continued interest in chiral amines, their synthesis remains challenging. Three metal-free catalytic methodologies available for the reduction of carbon–nitrogen double bond will be discussed: i) binaphthol-derived phosphoric acids catalyzed reductions, with dihydropyridine-based compound as the reducing agent; ii) trichlorosilane mediated reductions, in the presence of catalytic amounts of chiral Lewis bases; iii) metal-free hydrogenation of imines through FLP (Frustrated Lewis Pair) methodology, that involves the use of a combination of a strong Lewis acid with a variety of sterically encumbered Lewis bases, for examples phosphines or tertiary amines, to activate hydrogen at ambient conditions. Special attention will be devoted to the most recent applications of the last five years.

 Please wait while we load your content...
Please wait while we load your content...