Long-term, stable, and improved oxygen-reduction performance of titania-supported PtPb nanoparticles†
Abstract
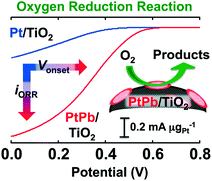
The long-term, stable, and improved electrocatalytic performance towards the oxygen reduction reaction (ORR) has been achieved by anatase-type titania (TiO2)-supported PtPb nanoparticles (NPs). Organometallic precursors, H2PtCl6·6H2O and Pb(CH3COO)2 were co-reduced using sodium borohydride at ambient temperature to precipitate the PtPb NPs (NiAs-type structure, P63/mmc, a = 0.4259 nm; c = 0.5267 nm, average particle size: 3 nm) over the TiO2 support (PtPb/TiO2). PtPb/TiO2 showed substantial electrocatalytic activity towards ORR both in terms of the onset potential and the mass activity compared to TiO2-supported Pt NPs (Pt/TiO2). The onset potential of PtPb/TiO2 was shifted to a higher electric potential by 180 mV compared to Pt/TiO2. Also, PtPb/TiO2 showed a seven-times higher ORR mass activity than Pt/TiO2. Neither the onset potential nor the mass activity of PtPb/TiO2 was altered after ORR cycles, whereas those of commercially available Vulcan carbon-supported Pt NPs (Pt/VC; Pt loading: 20 wt%) were largely altered. The PtPb NPs were not agglomerated over the TiO2 support even after repeated cycles under the electric potential condition, as the result of the strong interactions between the PtPb NPs and the TiO2 support, but the Pt NPs were significantly agglomerated over the TiO2- and the Vulcan carbon-supports due to the weak interactions between the Pt NPs and the supports. PtPb/TiO2 can be a practical cathode material for direct fuel cells in terms of its long-term stability and enhanced catalytic performance.

 Please wait while we load your content...
Please wait while we load your content...