Theoretical study on the chemical fixation of carbon dioxide with propylene oxide catalyzed by ammonium and guanidinium salts
Abstract
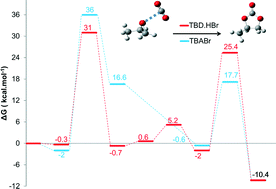
The cycloaddition of carbon dioxide onto propylene oxide catalysed by ammonium and guanidinium salts has been investigated using density functional theory (DFT) in order to understand the catalytic mechanism and the role of the cation and the anion. Two different possible pathways were considered, but it has been found that the one whereby the activation of the epoxide by the onium salt occurs before the addition of CO2 was consistent with the experimental findings. The rate-determining step was found to be the ring opening of the epoxide that results from the nucleophilic attack of the anion of the catalyst on the non-substituted carbon of the epoxide ring. Moreover, we found that in order to have an efficient catalyst, it is necessary to have an anion with an ambivalent nature (a high nucleophilicity and good leaving group ability). Finally, it turns out that for a given anion, the guanidinium salt is more efficient than the ammonium salt in opening the epoxide ring, thanks to the ability of the guanidinium cation to form hydrogen bonds with the oxygen of the epoxide.
- This article is part of the themed collection: Carbon dioxide conversion

 Please wait while we load your content...
Please wait while we load your content...