Modelling of catalytically oxidative decomposition of carbon tetrachloride on a ZnS nanocluster using density functional theory†
Abstract
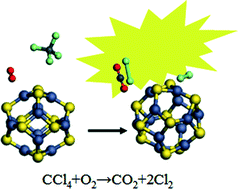
Using density functional theory (DFT), adsorption of O2 and co-adsorption of O2 and CCl4 on (ZnS)n clusters (n = 6, 7, 11, 12, 13, 15, 16, 17, 25, 26) and the mechanism of oxidative decomposition of CCl4 on a ZnS nanocluster were investigated. The decomposition of CCl4 without the ZnS cluster was also studied for comparison. The ZnS cluster achieved a significant catalytic effect during the oxidative decomposition of CCl4. It is found that CCl4 in gas phase undergoes a homolytic dissociation process with the activation by ZnS, reacting with O2 to produce a CCl3˙ radical and a ClOO˙ radical first. CO2 and Cl2 are identified as the final neutral reaction products, while COCl2, CO and Cl2O are produced as intermediates. The released energy of the total reaction, 149.83 kcal mol−1, is sufficient to afford the transition energy of the cataluminescence, based on which a novel and sensitive gas sensor for the determination of CCl4 could be constructed.

 Please wait while we load your content...
Please wait while we load your content...