Kinetics and catalyst deactivation in the enantioselective hydrogenation of ethyl benzoylformate over Pt/Al2O3†
Abstract
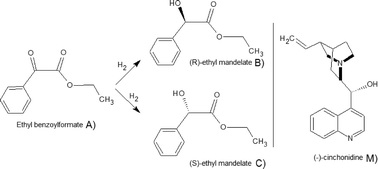
The enantioselective hydrogenation of ethyl benzoylformate (EBF) to (R)-ethyl mandelate over (−)-cinchonidine (CD)-modified Pt/Al2O3 catalyst in toluene in a semi-batch reactor was studied as a function of modifier concentration, reaction temperature, stirring rate and catalyst particle size. The kinetic results showed higher enantioselectivity and lower initial rates tending asymptotically to a constant value as the amount of modifier increased. The maximum enantiomeric excess (ee) and the initial hydrogenation rate or turnover frequency obtained under optimized conditions with an initial concentration of 5.6 mmol L−1 EBF at 25 °C over 5% (w/w) Pt/Al2O3 were 85% (modifier-to-surface Pt of 1.28) and 109 h−1 (modifier-to-surface Pt of 0.16), respectively. Additionally, catalyst deactivation due to the presence of impurities in the feed was prominent in some cases; therefore activated carbon was used as a cleaning agent of the raw material to remove impurities prior to catalyst addition.

 Please wait while we load your content...
Please wait while we load your content...