Peptidomimetics via modifications of amino acids and peptide bonds†
Abstract
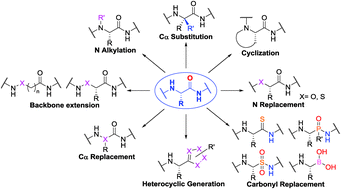
Peptidomimetics represent an important field in chemistry, pharmacology and material science as they circumvent the limitations of traditional peptides used in therapy. Self-structural organizations such as turns, helices, sheets and loops can be accessed by chemical modifications of amino acids or peptides. In-depth structural and conformational analysis and structure–activity relationships (SAR) offer a way to establish peptidomimetic libraries. Herein, we review recent developments in peptidomimetics that are formed via heteroatom replacement within the native amino acid backbone. Each sub-section describes structural features, utility and preparative methods.

 Please wait while we load your content...
Please wait while we load your content...