Synthesis of heterocycles via transition-metal-catalyzed hydroarylation of alkynes
Abstract
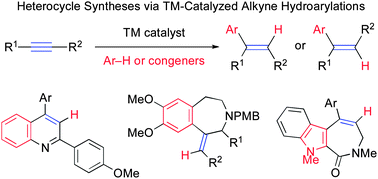
Transition-metal (TM)-catalyzed hydroarylation reactions of alkynes have received much attention, because they enable the net insertion of alkyne C–C triple bonds into C–H bonds of aromatic precursors, resulting in regio- and stereo-selective formation of synthetically useful arylalkenes. Taking advantage of this feature, TM-catalyzed alkyne hydroarylations have been successfully used for the synthesis of heterocycles. TM-catalyzed alkyne hydroarylations can be classified into three major categories depending on the type of reaction and precursors involved: (1) palladium-catalyzed reductive Heck reactions of alkynes with aryl halides, (2) TM-catalyzed conjugate arylation reactions of activated alkynes with arylboronic acids, and (3) TM-catalyzed aromatic C–H alkenylations with alkynes. This review surveys heterocycle synthesis via TM-catalyzed hydroarylation of alkynes according to the above classification, with an emphasis on the scope and limitations, as well as the underlying mechanisms.

 Please wait while we load your content...
Please wait while we load your content...