The shape of d-glucosamine†
Abstract
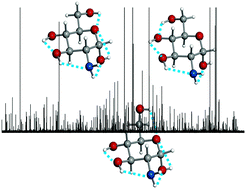
The bioactive amino monosaccharide D-glucosamine has been generated in the gas phase via laser ablation of D-glucosamine hydrochloride. Three cyclic α-4C1 pyranose forms have been identified using Fourier transform microwave techniques. Stereoelectronic hyperconjugative forces – essentially linked with the anomeric or gauche effect – and cooperative OH⋯O, OH⋯N and NH⋯O chains, extended along the entire molecule, are found to be the main factors driving the conformational behavior. The orientation of the NH2 group within each conformer has been determined by the values of the nuclear quadrupole coupling constants. The results have been compared with those recently obtained for the archetypical D-glucose.

 Please wait while we load your content...
Please wait while we load your content...