Semiconductor–redox catalysis promoted by metal–organic frameworks for CO2 reduction†
Abstract
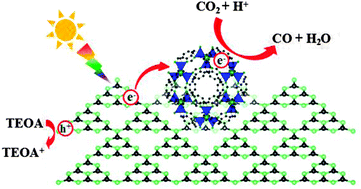
A noble-metal-free system for photochemical reduction of CO2 has been developed by integrating graphitic carbon nitride (g-C3N4) with a cobalt-containing zeolitic imidazolate framework (Co-ZIF-9). g-C3N4 acts as a semiconductor photocatalyst, whereas Co-ZIF-9 is a cocatalyst that facilitates the capture/concentration of CO2 and promotes light-induced charge separation. The two materials cooperate efficiently to catalyze CO2-to-CO conversion upon visible light illumination under mild reaction conditions. A 13C-labelled isotropic experiment proved that CO2 is the carbon source of the produced CO. Even without noble metals, the system still achieved an apparent quantum yield of 0.9 percent. The system displayed high photocatalytic stability, without noticeable alterations in the chemical and crystal structures of g-C3N4 and Co-ZIF-9 after the reaction.

 Please wait while we load your content...
Please wait while we load your content...