Pinacyanol chloride forms mesoscopic H- and J-aggregates in aqueous solution – a spectroscopic and cryo-transmission electron microscopy study†
Abstract
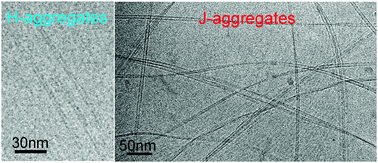
The aggregation behaviour of the cationic pinacyanol chloride in aqueous solution is investigated using absorption and linear dichroism spectroscopies, optical microscopy and cryogenic transmission electron microscopy (cryo-TEM). The investigations are focused on solutions in a concentration range from 50 μM up to 1 mM. At a concentration of 0.7 mM H-aggregates are detected that are characterized by a broad absorption band centred at ∼511 nm. The aggregates possess a tubular architecture with a single-layer wall thickness of ∼2.5 nm and an outer diameter of ∼6.5 nm. Linear dichroism spectroscopy indicates that the molecules are packed with their long axis parallel to the tube axis. These H-aggregates are not stable, but transform into J-aggregates on the time scale of weeks. The kinetics of J-aggregation depends on the dye concentration and the route of sample preparation, but can also be enhanced by shear stress. J-aggregates possess a split absorption spectrum composed of two longitudinally polarized J-bands and one H-band that is polarized perpendicular to the aggregate axis. The J-aggregates are ∼9 nm wide and several micrometer long fibrils consisting of stacked pairs of ribbons with a dumbbell-shaped density cross-section. Upon aging these ribbons laterally stack face-to-face to form tape-like aggregates.

 Please wait while we load your content...
Please wait while we load your content...