NO oxidation catalysis on copper doped hexagonal phase LaCoO3: a combined experimental and theoretical study†
Abstract
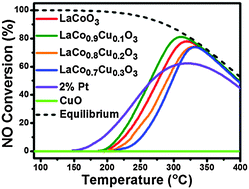
Cobalt-based perovskite catalysts showed excellent performance towards NO–NO2 oxidation. We systematically investigated the influence of different levels of Cu-doping on the catalytic performance of hexagonal phase LaCoO3 (LaCo1−xCuxO3 (x = 0.1, 0.2, 0.3)) for NO oxidation. The catalytic activities of the oxide catalysts followed the sequence: LaCo0.9Cu0.1O3 > LaCoO3 > LaCo0.8Cu0.2O3 > LaCo0.7Cu0.3O3 where the highest NO conversion for LaCo0.9Cu0.1O3 was 82% at 310 °C. The relevant structural characterizations were conducted by XRD, BET, FTIR and TEM. The interaction between Co and Cu promoted the conversion of NO to NO2. Upon increasing the Cu doping content, a decrease of the performance resulted from the generation of isolated CuO on the surface of the oxides, confirmed using H2-TPR and XPS. Combined with first-principle calculations, we explored the reaction mechanism of NO oxidation on the surface and found that Cu doping would facilitate the reaction by decreasing the energy of oxygen vacancy formation and the NO2 desorption barrier from Co- or Cu-nitrite.

 Please wait while we load your content...
Please wait while we load your content...