Adsorption studies of a phosphonic acid on ITO: film coverage, purity, and induced electronic structure changes†
Abstract
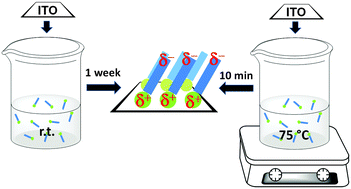
The deposition of a prototypical phosphonic acid from an ethanol solution onto indium-tin oxide is studied using XPS to assess the chemisorption kinetics and the purity of the film deposited, and UPS is employed to determine the electronic structure changes induced by the modifier. Room temperature (r.t.) vs. 75 °C depositions are compared, as well as the effect of using air plasma (AP) pretreatment vs. utilizing only detergent–solvent cleaning (DSC) prior to immersion. It is concluded that the order of adsorption kinetics and film purity follows the trend AP 75 °C > DSC 75 °C > AP r.t. > DSC r.t., while the work function obtained for each immersion time depends on whether the substrate is plasma pretreated or not and on the molecular dipole, which saturates at high coverages.

 Please wait while we load your content...
Please wait while we load your content...