Assessing capability of semiconductors to split water using ionization potentials and electron affinities only†
Abstract
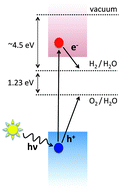
We show in this article that the position of semiconductor band edges relative to the water reduction and oxidation levels can be reliably predicted from the ionization potentials (IP) and electron affinities (AE) only. Using a set of 17 materials, including transition metal compounds, we show that accurate surface dependent IPs and EAs of semiconductors can be computed by combining density functional theory and many-body GW calculations. From the extensive comparison of calculated IPs and EAs with available experimental data, both from photoemission and electrochemical measurements, we show that it is possible to sort candidate materials solely from IPs and EAs thereby eliminating explicit treatment of semiconductor/water interfaces. We find that at pH values corresponding to the point of zero charge there is on average a 0.5 eV shift of IPs and EAs closer to the vacuum due to the dipoles formed at material/water interfaces.

 Please wait while we load your content...
Please wait while we load your content...