The structural and energetic aspects of substrate binding and the mechanism of action of the DapE-encoded N-succinyl-l,l-diaminopimelic acid desuccinylase (DapE) investigated using a hybrid QM/MM method†
Abstract
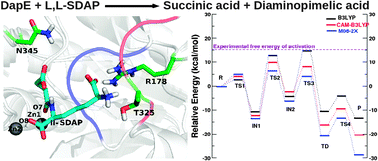
With increasing cases of fatal bacterial infections and growing antibiotic resistance, unrelenting efforts are necessary for identification of novel antibiotic targets and new drug molecules. The dapE-encoded N-succinyl-L,L-diaminopimelic acid desuccinylase (DapE) is a di-nuclear Zn containing enzyme in the lysine biosynthetic pathway which is indispensable for bacterial survival and absent in the human host, thus a potential antibiotic target. The DapE enzyme catalyzes the hydrolysis of N-succinyl-L,L-diaminopimelic acid (SDAP) to give rise to succinic acid and L,L-diaminopimelic acid. The mechanism of action of the DapE catalyzed SDAP hydrolysis is investigated employing a hybrid QM/MM computational method. The DapE side chains, such as, Arg178, Thr325, Asn345, are found to play a role in substrate identification and stabilization of the enzyme active site. Furthermore, a glycine rich loop (Gly322–Ser326) is found to facilitate tight binding of the substrate in the enzyme active site. The catalytic reaction progresses via a general acid–base hydrolysis mechanism where Glu134 first acts as a Lewis base by activating the catalytic water molecule in the active site, followed by guiding the resulting hydroxyl ion for a nucleophilic attack on the substrate, and finally acts as a Lewis acid by donating a proton to the substrate. The intermediates and transition states along the reaction pathway have been structurally and energetically characterized. A conformational change in the side chain of Asp100, which bridges the two Zn centers of the enzyme, is observed which facilitates the enzymatic action by lowering the activation energy and leads to the formation of a new intermediate during the catalytic reaction. The nucleophilic attack is found to be the rate determining step.

 Please wait while we load your content...
Please wait while we load your content...