Cu(ii)–alkyl amine complex mediated hydrothermal synthesis of Cu nanowires: exploring the dual role of alkyl amines
Abstract
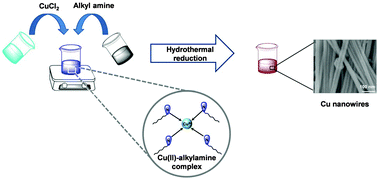
Ligands/surfactants play an important role in the synthesis of anisotropic nanomaterials. Other than site specific binding to the crystal plane, they can also undergo complexation with metal ions, altering the nature of the metal complex. The ligand–metal complex formation could be sufficient to modify the reaction kinetics and could affect the size and morphology of the nanostructures. In this article, we investigated such a change in the metal precursor caused by ligands (i.e., alkyl amines) in the hydrothermal synthesis of Cu nanowires in the presence of glucose as a reducing agent. Comparative studies were carried out with other nitrogen-based surfactants such as cetyl trimethyl ammonium bromide and polyvinyl pyrrolodine. Our experimental results confirmed the complex formation of Cu2+ ions with alkyl amines and its application for nanowire synthesis. Slow reduction of this complex allows for the generation of twinned seeds, which are later grown into nanowires by consuming newly generated seeds in the presence of excess alkyl amine.

 Please wait while we load your content...
Please wait while we load your content...