Assessing the impact of anion–π effects on phenylalanine ion structures using IRMPD spectroscopy†
Abstract
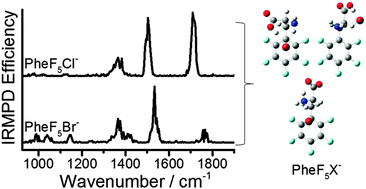
The gas-phase structures of two halide-bound phenylalanine anions (PheX−, X = Cl− or Br−) and five fluorinated derivatives have been identified using infrared multiple photon dissociation (IRMPD) spectroscopy. The addition of electron-withdrawing groups to the aromatic ring creates a π-acidic system that additionally stabilizes the halide above the ring face. Detailed ion structures were determined by comparing the IRMPD spectra with harmonic and anharmonic infrared spectra computed using B3LYP/6-311++G(d,p) as well as with 298 K enthalpies and Gibbs energies determined by the MP2(full)/6-311++G(2d,2p)//B3LYP/6-311++G(d,p) and MP2(full)/aug-cc-pVTZ//B3LYP/6-311++G(d,p) methods. PheX− structures were found to be dependent on both the nature of the anion and the extent of ring fluorination. Canonical isomers were established to be the dominant structures in every case, but halide addition significantly narrowed the energy gap with zwitterionic potential energy surfaces. This enabled zwitterions to appear as minor contributors to the gas-phase populations of Phe35F2Cl− and PheF5Br−.

 Please wait while we load your content...
Please wait while we load your content...