The structures and thermodynamic stability of copper(ii) chloride surfaces†
Abstract
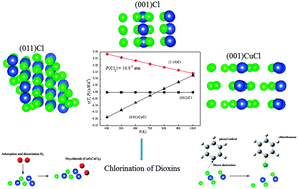
Using density functional theory calculations of periodic slabs, within the generalised gradient approximation, this study provides optimised structures for all plausible terminations of copper(II) chloride surfaces along the three low-index orientations. The ab initio atomistic thermodynamic approach serves to construct a thermodynamic stability diagram for CuCl2 configurations as a function of the chemical potential of chlorine (ΔμCl(T,P)). We observe a shift in thermodynamic stability ordering at around ΔμCl(T,P) = −1.0 eV between a copper–chlorine terminated (001) surface (i.e., (001)CuCl) and a (001) chlorine-covered surface (i.e., (001)Cl). This conclusion accords with experimental observations that report CuCl-bulk like structures, acting as a prerequisite for the formation of CuCl2-bulk like arrangements in the course of copper chlorination. Profound stabilities and optimised structures of (001)CuCl and (001)Cl configurations are discussed within the context of the functionality of CuCl2 as the chief chlorination and condensation catalyst of aromatic pollutants under conditions relevant to their formation in thermal systems, i.e. 400–1000 K, a total operating pressure of 1.0 atm and PCl2 = 10−6–10−4 atm (1.0–100.0 ppm).

 Please wait while we load your content...
Please wait while we load your content...