Directed precipitation of hydrated and anhydrous magnesium carbonates for carbon storage
Abstract
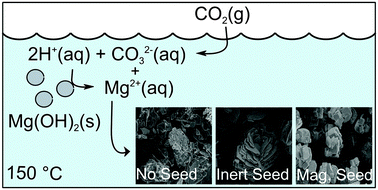
Magnesite is the most desirable phase within the magnesium carbonate family for carbon storage for a number of reasons: magnesium efficiency, omission of additional crystal waters and thermodynamic stability. For large-scale carbonation to be a viable industrial process, magnesite precipitation must be made to occur rapidly and reliably. Unfortunately, the formation of metastable hydrated magnesium carbonate phases (e.g. MgCO3·3H2O and Mg5(CO3)4(OH)2·4H2O) interferes with the production of anhydrous magnesite under a variety of reaction conditions because magnesite crystals are slower to both nucleate and grow compared to the hydrated carbonate phases. Furthermore, the reaction conditions required for the formation of each magnesium carbonate phases have not been well understood with conflicting literature data. In this study, the effects of both magnesite (MgCO3) and inert (Al2O3) seed particles on the precipitation of magnesium carbonates from a Mg(OH)2 slurry were explored. It was interesting that MgCO3 seeding was shown to accelerate anhydrous magnesite growth at temperatures (80–150 °C), where it would normally not form in short time scale. Since the specific surface areas of MgCO3 and Al2O3 seeding particles were similar, this phenomenon was due to the difference in the surface chemistry of two seeding particles. By providing a template with similar chemistry for the growth of magnesite, the precipitation of anhydrous magnesite was demonstrated. The effect of temperature on seeded carbonation was also investigated. A comparison with published MgCO3 precipitation rate laws indicated that the precipitation of magnesite was limited by either CO2 adsorption from the gas phase or the dissolution rate of Mg(OH)2.

 Please wait while we load your content...
Please wait while we load your content...