Thermodynamic analysis of framework deformation in Na,Cs-RHO zeolite upon CO2 adsorption†
Abstract
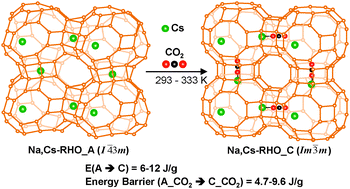
Fully dehydrated and partially sodium-cesium containing RHO zeolite (Na,Cs-RHO) shows a genuine inflection in the CO2 isotherms in the temperature range 293–333 K that can be attributed to a sorbate-induced framework deformation from an acentric (A) to a centric (C) phase due to a partial cation rearrangement. This peculiar sorption pattern can be captured by the formulation of thermodynamic isotherms, providing a direct enthalpic and entropic signature of the CO2 adsorption–desorption process during deformation. Using this formulation, the energy barrier between the acentric and centric phases for CO2 adsorption–desorption was estimated in the range 4.7–9.6 J g−1 of solid (15–32 kJ mol−1), reflecting a higher CO2 affinity for the acentric phase, whereas the elastic energy involved during framework distortion was estimated in the range 6–12 J g−1 of solid (19–39 kJ mol−1) with a relative maximum at 303 K and showing a dominant entropic contribution.

 Please wait while we load your content...
Please wait while we load your content...