Experimental and first-principles study of guanine adsorption on ZnO clusters
Abstract
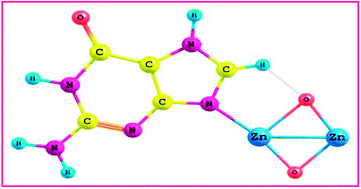
Theoretical investigation of guanine, DNA base adsorption on the ZnO model clusters, viz., Zn2O2, Zn3O3, Zn4O4 ring (R) and Zn4O4 wurtzite (W) in terms of geometry, binding site, binding energy (EB), energy gap (Eg), electronic and spectral properties were studied by a density functional theory (DFT) method. The guanine adsorption on the ZnO (G–ZnO) clusters is modeled by the B3LYP/LanL2DZ method. The calculated binding energy (EB) and energy gap (Eg) of the guanine molecule are highly dependent on the nature of the cluster size and vary with the size of the clusters. Physisorption proceeded via formation of the N⋯Zn bond between guanine and the active Zn2+ site on ZnO. The HOMO–LUMO energies show that charge transfer occurs in the G–ZnO clusters, from ZnO to guanine to better understand the interaction. The Mulliken charges are computed. The electronic properties of ZnO and G–ZnO clusters were compared with different basis sets (B3LYP/6-31G, B3LYP/6-311G, MP2/6-31G and MP2/LanL2DZ). Experimental information like microscopic and spectroscopic evidence is also included for understanding the guanine–ZnO interactions. The G–ZnO composite was prepared by a precipitation method and characterized by SEM with EDX, FT-IR and FT-RAMAN analysis. The interaction of guanine with ZnO nanoparticles was observed by UV-vis spectroscopy. The experimental results are compared with the DFT results in the light of these new insights.

 Please wait while we load your content...
Please wait while we load your content...