New insights into the by-product fatigue mechanism of the photo-induced ring-opening in diarylethenes†
Abstract
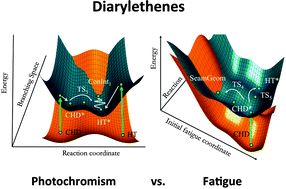
The photochromic properties of diarylethenes, some of the most studied class of molecular switches, are known to be controlled by non-adiabatic decay at a conical intersection seam. Nevertheless, as their fatigue-reaction mechanism – leading to non-photochromic products – is yet to be understood, we investigate the photo-chemical formation of the so-called by-product isomer using three complementary computational methods (MMVB, CASSCF and CASPT2) on three model systems of increasing complexity. We show that for the ring-opening reaction a transition state on S1(2A) involving bond breaking of the penta-ring leads to a low energy S1(2A)/S0(1A) conical intersection seam, which lies above one of the transition states leading to the by-product isomer on the ground state. Therefore, radiationless decay and subsequent side-product formation can take place explaining the photo-degradation responsible for the by-product generation in diarylethene-type molecules. The effect of dynamic electron correlation and the possible role of inter-system crossing along the penta-ring opening coordinate are discussed as well.

 Please wait while we load your content...
Please wait while we load your content...