Aqueous production of oxygen atoms from hydroxyl radicals†
Abstract
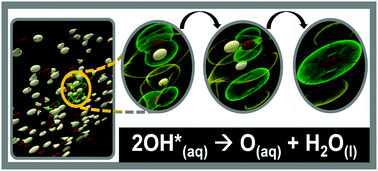
The behavior of the hydroxyl radical (OH*) in solution is significant to a broad range of scientific and technological fields. OH* is considered a highly reactive, short-lived species and previous studies have neglected the possibility of encounters of two OH* in solution. However, these encounters may be nonnegligible in environments with elevated local OH* concentrations, such as under many in vivo processes and within nuclear infrastructure. High concentrations of OH* in vivo are considered to be very dangerous; OH* has been related to many ailments ranging from cancer to Alzheimer's disease. Here we probe details of the reactions and interactions that can occur between two OH* in water by utilizing Car–Parrinello molecular dynamics simulations and advanced visualization techniques. The recombination reaction to form hydrogen peroxide is confirmed for the singlet electronic state. In contrast, the triplet state yields an oxygen atom, O(aq). This species has been previously detected in experimental water-radiolysis studies, but its origin could not be determined. O(aq) is a much more potent biradical than its parent OH* and its presence can impact many in vivo processes. This study also reveals that the hemibonded interaction plays key role in the behavior of OH*(aq). Our findings have major implications to the scientific understanding of the impacts of high local OH* concentrations, such during oxidative stress and in aging processes. Given its importance, this study will form the basis of further experimental and theoretical investigations exploring the role of O(aq) in a number of contexts.

 Please wait while we load your content...
Please wait while we load your content...