Direct synthesis of phosphorus and nitrogen co-doped monolayer graphene with air-stable n-type characteristics†
Abstract
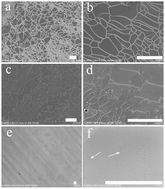
Large-area substitutional phosphorus–nitrogen co-doped monolayer graphene is directly synthesized on a Cu surface by chemical vapor deposition using molecules of phosphonitrilic chloride trimer as the phosphorus and nitrogen sources. The doping levels of both phosphorus and nitrogen atoms decrease as a function of the growth temperature. In contrast, the doping effect is enhanced with temperature because of the formation of more stable bond configurations for dopants at higher temperatures. Moreover, the doping amount of nitrogen atoms is always higher than that of phosphorus atoms at all used temperatures. The phosphorus and nitrogen co-doped graphene exhibits remarkable air-stable n-type characteristics. This work demonstrates the critical role of phosphorus atoms in achieving enhanced electron donation compared to nitrogen atom doping of graphene, and is important for various applications associated with the need for air-stable n-type graphene materials.

 Please wait while we load your content...
Please wait while we load your content...