Stabilization mechanism of electrodeposited silicon thin films
Abstract
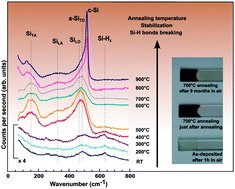
Amorphous composite silicon thin films electrodeposited in tetrahydrofuran, containing up to 80 at% of Si and exhibiting an homogeneous dispersions of O, C and Cl in the amorphous Si matrix, have been successfully stabilized against oxidation using a post-annealing step in inert atmosphere. In order to understand the impact of the annealing step on their stabilization against oxidation, their composition and structure have been investigated upon heat treatments. It has been shown that the presence of impurities such as O, C and Cl does not have any impact on the stabilization process, which is rather linked to the presence of hydrogen in the Si composites. This conclusion has been drawn after a detailed analysis of the bonding structure of films annealed at different temperatures and dwell times by the mean of Raman spectroscopy. It has been shown that annealing the as-deposited films at 350 °C for a couple of hours or at higher temperatures induced a hydrogen evolution, characterized by the breaking of Si–H bonds and the formation of Si–Si bonds, which stabilized the silicon network. The understanding and the reproducibility of this stabilization process of silicon thin film electrodeposited in organic solvent paves the way for their use for many applications.

 Please wait while we load your content...
Please wait while we load your content...