Atmospheric formation of the NO3 radical from gas-phase reaction of HNO3 acid with the NH2 radical: proton-coupled electron-transfer versus hydrogen atom transfer mechanisms†
Abstract
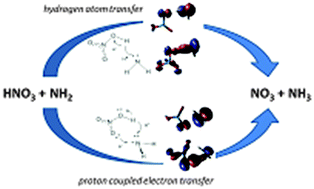
The gas-phase reaction of nitric acid with the amidogen radical under atmospheric conditions has been investigated using quantum mechanical (QCISD and CCSD(T)) and DFT (B3LYP, BH&HLYP, M05, M05-2X, and M06-2X) calculations with the 6-311+G(2df,2p), aug-cc-pVTZ, aug-cc-pVQZ and extrapolation to the CBS basis sets. The reaction begins with the barrierless formation of a hydrogen-bonded complex, which can undergo two different reaction pathways, in addition to the decomposition back to the reactants. The lowest energy barrier pathway involves a proton-coupled electron-transfer mechanism, whereas the highest energy barrier pathway takes place through a hydrogen atom transfer mechanism. The performance of the different DFT functionals in predicting both the geometries and relative energies of the stationary points investigated has been analyzed.

 Please wait while we load your content...
Please wait while we load your content...