Water and carbon oxides on monoclinic zirconia: experimental and computational insights†
Abstract
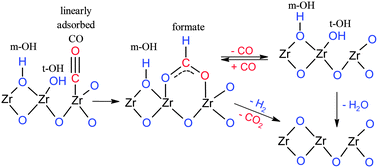
Zirconium oxide (ZrO2, zirconia) is an interesting catalytic material to be used in biomass conversion, e.g., gasification and reforming. In this work, we show that reducing and hydrating pretreatments affect the surface sites on monoclinic zirconia. The multitechnique approach comprises temperature-programmed surface reactions (TPSR) under CO and CO2 at 100–550 °C, in situ DRIFTS investigations of the surface species and density functional theory (DFT) calculations. The key findings of the work are: (1) formates are formed either directly from gas-phase CO on terminal surface hydroxyls or via the linear CO surface species that are found exclusively on the reduced zirconia without water treatment; (2) formates are able to decompose at high temperature either reversibly to CO or reductively to CO2 and H2via surface reaction between formates and multicoordinated hydroxyls; and (3) a new weak reversible binding state of CO is found exclusively on ZrO2 that is first reduced and subsequently hydrated.


 Please wait while we load your content...
Please wait while we load your content...