Direct observation of OH formation from stabilised Criegee intermediates†
Abstract
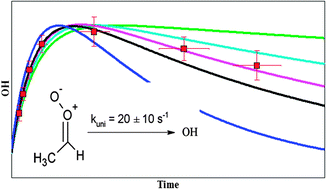
The syn-CH3CHOO Criegee intermediate formed from the ozonolysis of propene and (E)-2-butene was detected via unimolecular decomposition and subsequent detection of OH radicals by a LIF-FAGE instrument. An observed time dependent OH concentration profile was analysed using a detailed model focusing on the speciated chemistry of Criegee intermediates based on the recent literature. The absolute OH concentration was found to depend on the steady state concentration of syn-CH3CHOO at the injection point while the time dependence of the OH concentration profile was influenced by the sum of the rates of unimolecular decomposition of syn-CH3CHOO and wall loss. By varying the most relevant parameters influencing the SCI chemistry in the model and based on the temporal OH concentration profile, the unimolecular decomposition rate k (293 K) of syn-CH3CHOO was shown to lie within the range 3–30 s−1, where a value of 20 ± 10 s−1 yields the best agreement with the CI chemistry literature.

 Please wait while we load your content...
Please wait while we load your content...